Accreditations
Food and Agricultural – ISO/IEC 17025 Testing Laboratory Accreditation
What is Food and Agricultural – ISO/IEC 17025 Testing Laboratory Accreditation?
The ANAB testing laboratory accreditation program is designed to promote confidence in testing supporting the food and agricultural industry and improve quality through assuring compliance with international standard ISO/IEC 17025 and applicable government and industry requirements..
Our goal through accreditation is increased acceptance of testing across the food and agricultural industry and improve the quality of testing laboratories and the data they produce. ANAB accreditation has a long history of acceptance across the food and agricultural industry including government and regulatory authorities, food manufactures, and food retailers.
Accreditation promotes confidence in test results and will help move your testing laboratory toward acceptance in the food and agricultural industry. Accreditation will provide a competitive advantage by increasing the quality of test results and supporting consistent testing laboratory operations.
ANAB accreditation managers and testing experts have practical experience in the field and are often considered peers to the laboratories. The ANAB food and agricultural laboratory accreditation program is well-established nationally and internationally and recognized by many state and federal agencies and should be your choice for accreditation.
Steps to Accreditation
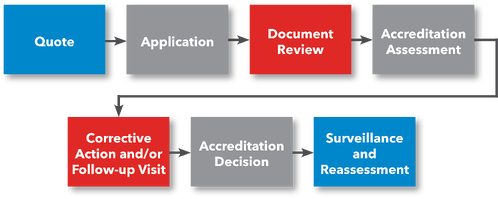
Steps for Getting Accredited
- Request a Quote
- File Application
- Prepare for Accreditation Assessment
- Submit Documentation for Review
- (Optional) Preliminary Assessment
- Accreditation Assessment
- Corrective Action (if applicable)
- ANAB Accreditation Decision
- Receive Accreditation Certificate
ISO/IEC 17025
General Requirements for the Competence of Testing and Calibration Laboratories
ISO/IEC 17025 specifies the general requirements for the competence, impartiality and consistent operation of laboratories.
ISO/IEC 17025 is applicable to all organizations performing laboratory activities, regardless of the number of personnel.
Laboratory customers, regulatory authorities, organizations and schemes using peer-assessment, accreditation bodies, and others use ISO/IEC 17025 in confirming or recognizing the competence of laboratories.
Talk to an Expert
Keith Klemm
Senior Manager of Accreditation, Inspection, Laboratories, and Related Activities
414-501-5343

Need Training To Support Your Accreditation Journey?
Learn at your own pace with online courses or choose an instructor led class offered online or in a convenient location.

